The future of clinical research is inclusive
Collaboration and patient-centered innovation are creating real impact for communities.

By Quita Beeler Highsmith
Vice president and chief diversity officer, Genentech
July 24th, 2024
In recent years, the biotech industry has revolutionized treatment for some of the world’s most complex diseases through cutting-edge drug discovery and development. As we celebrate these advances, we must also acknowledge that far too many communities have been excluded from the clinical research fueling these innovations.
Fewer than 20 percent of new drugs between 2014 and 2021 had clinical study data on benefits or side effects for Black patients.1 And nearly 40 percent of clinical studies between 2000 and 2020 that reported race or ethnicity data did not mention Hispanic/Latinx enrollment.2 This is unacceptable. To drive better outcomes for all patients, we have to start asking bigger and bolder questions. We must actively identify and eliminate barriers to clinical research participation to build trust and ensure our medicines are safe and effective for everyone who needs them.
At Genentech, we established our cross-functional Advancing Inclusive Research® (AIR) initiative in 2017 to address these barriers. Since then, we’ve been working to embed inclusive clinical research practices into all of our studies, but we know that one company cannot solve this challenge alone. In 2021, we launched the groundbreaking AIR Site Alliance, a first-of-its-kind coalition of clinical research sites that collaborate to improve the enrollment of historically underrepresented communities in clinical studies and establish best practices that can be leveraged industry wide.
Three years later, here’s what we’ve learned.
Lesson 1: To reach patients, meet them where they are
We can’t just go into communities when we need them. Proactively embedding ourselves as trusted and accessible partners helps us reach more communities with the information and resources they need to be able to participate in clinical research. We also have to deepen our understanding of the healthcare experiences of the patient communities we’re aiming to reach so we can repair justifiable medical mistrust.

Through our AIR Site Alliance, we are doing just that. Over the past three years, we have seen that patients of color do want to participate in clinical research, despite harmful myths that suggest otherwise. And we have the data to prove it.
Each of our Site Alliance partners is leveraging more inclusive study protocols as well as custom resources adapted to their communities’ needs, such as patient education materials and support, appropriate food and transportation stipends and healthcare provider bias training. As a result, the six oncology centers in the Site Alliance have enrolled 89 percent more Black and Hispanic/Latinx patients than their peers on the same studies, and the ophthalmology sites have enrolled 51 percent more of these underrepresented patient groups than their study peers. Collectively, they are enrolling these patients twice as fast as other sites on the same studies.
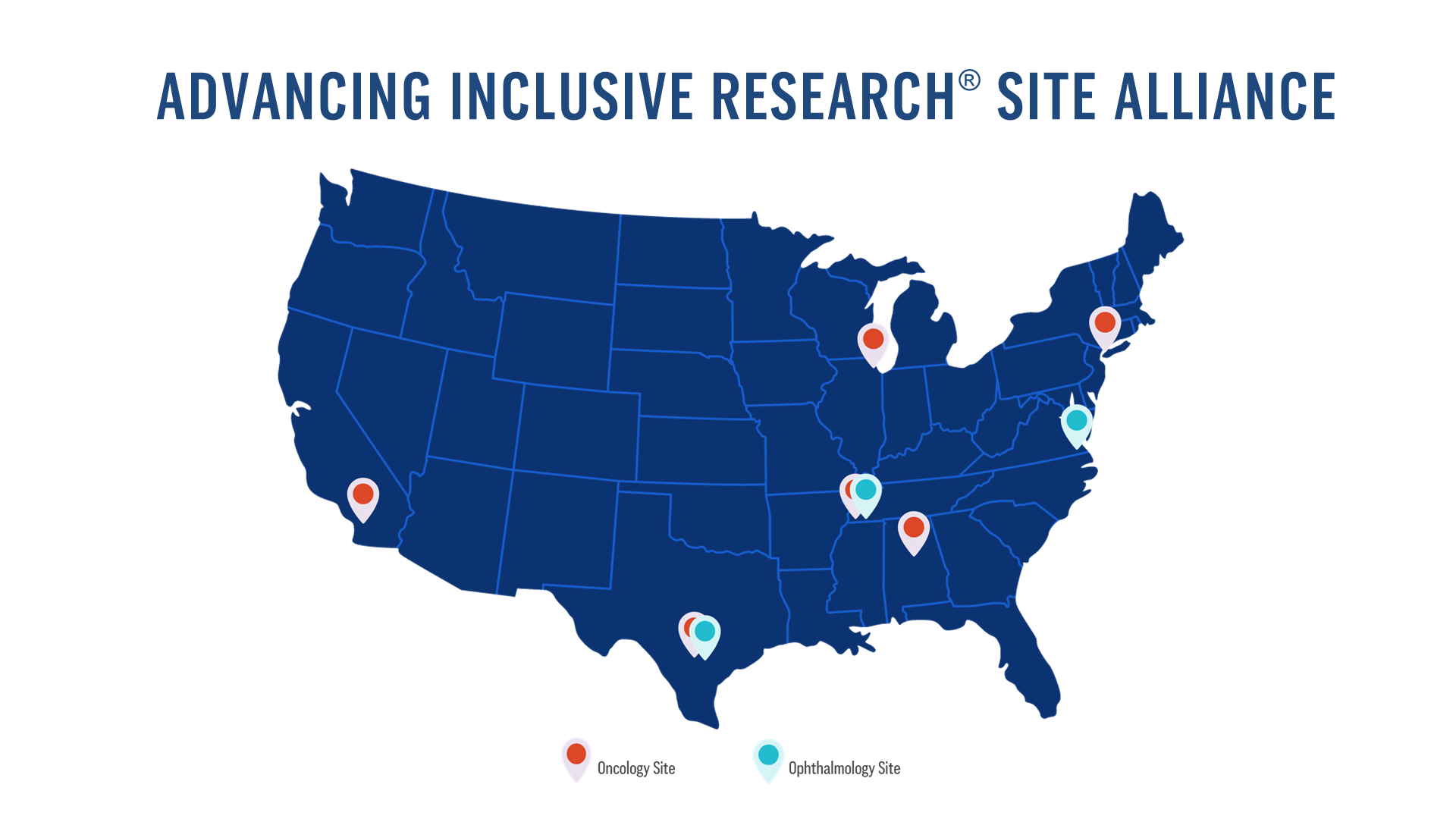
Our Advancing Inclusive Research Site Alliance includes nine sites across the country that prioritize inclusive research to increase the representation of diverse patient populations in oncology and ophthalmology clinical studies. Learn more about each site here.
Lesson 2: Learning and adapting drives progress
As I like to say, you must measure what you treasure. This has resulted in a data-driven approach to inclusive research at Genentech, in which we document and assess our efforts and adapt based on what has and hasn’t worked.
This approach has put us ahead of the curve. For example, in 2020, we asked our research teams to begin incorporating inclusive research action plans into their study design process. Two years later, the FDA shared guidance, now mandated by legislation, requiring that sponsors of Phase 3 clinical studies submit Diversity Action Plans (DAPs) outlining how their study designs will increase enrollment of underrepresented communities beginning in 2025. We were well-positioned to meet and exceed these expectations because of our strong inclusive research infrastructure, underscoring my belief that you don’t have to get ready if you stay ready. By December 2023, 76 DAPs had been submitted to the FDA across the biopharma industry. Genentech submitted 20 last year alone.
Even with years of inclusive research experience behind us, we don’t pretend to have all the answers. Through the Site Alliance and beyond, we’re continuing to collaborate with community-based organizations, researchers and companies across our industry to pilot untested approaches, asking big questions to find better solutions.
Lesson 3: You need to be in it for the long haul
For too long, people of color have been left out of clinical research that directly impacts their healthcare access, experiences and outcomes. We have an opportunity and an obligation to bring them into clinical research if we’re to truly advance health equity. Doing so will require serious time and investment. We can’t start today and hope for results tomorrow; the kind of systemic change we’re looking for takes time.
Genentech’s progress reflects the culmination of our efforts to embed diversity, equity and inclusion into all aspects of our business over the last two decades – and we’re still learning, evolving our approaches, applying what we’ve learned and repeating this process. We’re in it for the long haul.
I share these lessons so that others working to drive better patient outcomes can join us in creating a blueprint for inclusive research to spur change and make health equity a reality.
Genentech strives to broaden racial and ethnic enrollment in clinical studies to ensure treatments are safe and effective for all.
Sources:
1 Green, AK, Trivedi, N, Hsu, JJ, et al. Despite the FDA’s five-year plan, Black patients remain inadequately represented in clinical trials for drugs. Health Affairs. 2022;41(3).
2Turner, BE, Steinberg JR, Weeks, BT, et al. Race/ethnicity reporting and representation in US clinical trials: A cohort study. The Lancet Regional Health: Americas. 2022;11:100252.
The content is paid for and supplied by advertiser. The Washington Post was not involved in the creation of this content.